News
-

CorestemChemon Advances Toward ALS Stem Cell Commercialization with Regulatory Progress in Korea and NSF-Backed U.S. Expansion
2025.12.18
-
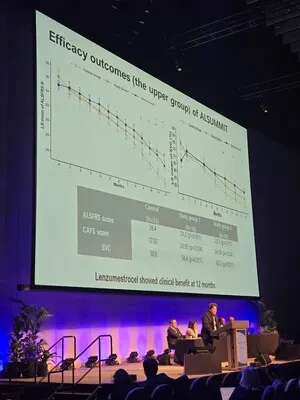
Neuronata-R® Phase 3 Results Confirm Subgroup Benefit at PACTALS 2025, Strengthening Biomarker-Driven Pathway
2025.09.18
-

Neuronata-R® Stem Cell Therapy Shows Promise in ALS Phase 3 Subgroup Analysis, Moves Toward FDA Accelerated Approval
2025.07.21
-

Neuronata-R(R) Stem Cell Therapy Shows Promise in ALS Phase 3 Subgroup Analysis, Moves Toward FDA Accelerated Approval
2025.06.09
-

CorestemChemon Partners with Leading U.S. ALS Patient Association to Promote in North American Market
2024.06.18
-

CorestemChemon ran in marathon on ALS Awareness Day for the people with ALS
2024.06.03
-

CorestemChemon Participate in '2024 JP Morgan Healthcare Conference'
2024.02.15
-

CorestemChemon to Participate in 'BIO Europe 2023'
2024.02.15
-

"CorestemChemon Invited to Korean-American Pharmaceutical & Bio Union Event to Present Plans for ‘Neuronata-R Phase III Clinical Trial' and 'Non-clinical CRO Business'"
2024.02.15

